23.5.2023: Melika Azim Zadegan & Catherine Suubi Kayonga: Insights from Neuro Ethics and Patient Rights Course
Melika Azim Zadegan & Catherine Suubi Kayonga: Insights from Neuro Ethics and Patient Rights Course
As researchers, our responsibilities extend beyond the realm of conducting valid and reliable research. We understand the significance of prioritising the well-being and rights of our study participants, as well as the potential impact of our work on society.
In pursuit of a more holistic approach to our multi/interdisciplinary research in neuroscience, the Neuro-Innovation Programme has designed a groundbreaking course that caters specifically for us doctoral researchers in this PhD Programme. This course, Neuro Ethics and Patient Rights, led by Professors Ville Leinonen and Anna Mäki-Petäjä-Leinonen, has equipped us with the knowledge and tools necessary to navigate the complex terrain of ethical considerations.
In this blog post, we invite you to embark on a collective journey with us as we share our experiences attending this course, exploring its valuable insights, and reviewing its practical applications.
What makes research ethical?
Have you ever wondered, “What makes research ethical?” Ethics in research serves as a guiding compass, determining what is deemed acceptable or unacceptable. It establishes the norms that align with research objectives and fosters values like mutual respect, trust, and accountability. Adhering to ethical guidelines enables researchers to minimise errors, safeguard data integrity, and foster a collaborative work environment.
Understanding the distinction between “good” and “bad” in research involves delving into personal values, grappling with ethical dilemmas, and evaluating various ethical theories. This comprehensive approach equips researchers with the necessary skills to navigate the intricate landscape of ethical decision-making.
How Do Established Frameworks and Regulations Safeguard Participants’ Well-being?
While every study aims to tackle important questions, it’s essential to acknowledge the potential risks and inconveniences it may pose to participants. As researchers, we have a responsibility to continuously assess our study activities, identifying and addressing any new risks that may arise.
Here, we review some established frameworks together that illuminate our journey into understanding ethical research:
- Belmont Report: Emphasizes principles of respect for individuals, beneficence, and justice, guiding researchers to treat participants with dignity and honour their autonomy.
- Declaration of Helsinki: Outlines ethical principles for research involving humans, emphasising the importance of considering factors such as research feasibility and social/clinical value to ensure benefits outweigh risks.
- General Data Protection Regulation (GDPR): sets guidelines regarding the collection, processing, and storage of personal data. Researchers are must comply with to these GDPR regulations when managing personal data, ensuring that they obtain informed consent, implement suitable data protection measures, and employ secure data storage systems. To safeguard participants’ personal information, methods such as anonymisation or pseudonymization should be employed. Additionally, regular updates to security measures must be performed to safeguard participants’ privacy.
- Institutional Review Boards (IRBs). In the field of biomedical research, there are regulations set by the Food and Drug Administration (FDA) that require the involvement of Institutional Review Boards (IRBs). These IRBs are responsible for reviewing and monitoring biomedical research involving human subjects. Their role is to protect the rights and well-being of participants. They conduct thorough assessments of research studies to determine the level of risk involved. Factors like the psychological well-being of participants and maintaining confidentiality are taken into consideration during this evaluation. Studies with higher risks require more comprehensive review processes and increased protections for the participants. By carefully evaluating research materials, such as informed consent documents and investigator brochures, the IRB ensures that ethical principles and guidelines are followed, prioritising the well-being and dignity of all those involved. The unwavering commitment of IRBs to upholding these standards contributes to the advancement of responsible and ethical biomedical research.
These principles not only guide researchers in making ethical decisions but also contribute to the overall integrity and credibility of the scientific community.
Seven Pillars of Ethical Research: The Helsinki Principles in Practice
Applying research in practice is a crucial aspect of conducting ethical research. Throughout our course, we explored the practical application of the seven main ethical principles outlined in the Declaration of Helsinki. In the realm of clinical research involving human subjects, it is imperative to adhere to these principles.
While understanding these principles is important, the true challenge lies in implementing them effectively.
Translating these ethical principles into practice requires careful consideration during every stage of the research process. It begins with incorporating these principles into the study design, ensuring the research is conducted in an ethical manner, and appropriately disseminating the findings. Upholding the highest standards of conduct is essential, encompassing obtaining informed consent from participants and safeguarding their privacy and confidentiality.
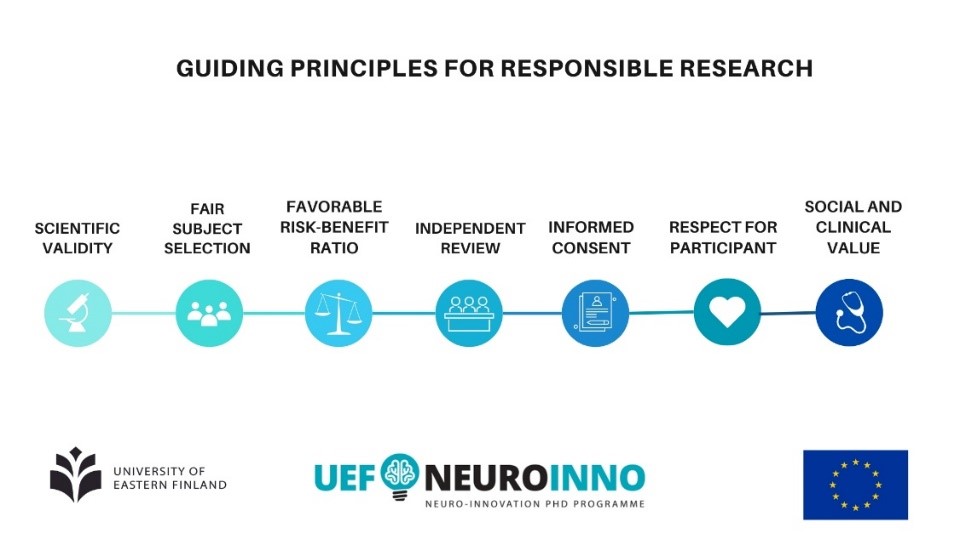
By adhering to these principles in the process of conducting a study involving humans, obtaining informed consent, protecting confidentiality, and carefully considering the potential benefits and risks of the research, researchers can ensure that participants’ rights are respected, their autonomy is upheld, and trust is established.
Insights and experiences from the participants
A research journey is like stepping into a room filled with mirrors, each reflecting different perspectives on ethics. Prior to the workshop, we were divided into groups, each comprising four doctoral researchers, to evaluate and provide feedback on each other’s research plans using the seven ethical principles mentioned earlier.
On the dedicated workshop day, we actively participated in discussions centered around our respective PhD project topics. This provided us with the opportunity to listen to individual comments and feedback from our colleagues regarding the ethical aspects of our research plans. These interactions and the practical application of ethical principles provided us with a deeper understanding of each other’s projects and the ethical considerations within different fields. Such skills are essential in ensuring responsible research practices across diverse settings and industries.
Through this collaborative process, we gained firsthand experience in incorporating ethical principles and recognized the importance of interdisciplinary collaboration. By evaluating research plans from an ethical standpoint, we were able to identify potential ethical issues and share our experiences with peers from various disciplines.

Let’s take a closer look at the experiences of attendees! Melika, doctoral researcher in Innovation Management at UEF Business School, believes that transferable skills play a vital role for PhD candidates and researchers as they can be applied across various roles and disciplines.
“These skills encompass problem-solving, communication, critical thinking, and adaptability. Attending courses like the Neuroethics and Patient Rights course can assist researchers in expanding their skillset and preparing for diverse career paths beyond academia.”
She notes how the skills acquired from the course can be transferable to future research projects too:
“As we progress in our research journeys, we recognize the critical role of incorporating ethical considerations at every stage of the process. This includes obtaining informed consent, safeguarding confidentiality and privacy, respecting participant rights, and meticulously balancing the benefits and risks associated with our research. We have embraced the responsibility of conducting ethical and responsible research that contributes to the greater good.”
Ahmed‘s experiences attending this course revealed the immense impact it had on his approach to research, particularly when it comes to prioritizing patient rights. Ahmad, a doctoral researcher from the faculty of Health Sciences at the A.I. Virtanen Institute for Molecular Sciences, passionately emphasises the need for a flexible compass that researchers can follow, providing them with common rules to ensure ethical conduct in their work. In Ahmad’s words.
“Everyone hears about ethics. However, almost everyone perceives it differently.”
He believes these rules should be regularly reviewed and adjusted to keep pace with evolving technology and challenges. Ahmed’s experience underscores the importance of dynamic ethical frameworks in maintaining credibility and sustainability across research fields. He also advocates for involving patients in research, emphasising the significance of safeguards and their rights. He believes that researchers, especially those starting their careers, should participate in courses like Neuro-ethics and Patient Rights.
“It’s a step… that enhances the credibility and sustainability of all research fields.”
Ahmed says. Catherine, from the faculty of Social Science, highlights the practical insights gained from the course. She emphasises the importance of tailoring ethics to different research contexts and understanding the fluidity of ethical interpretation and application. Catherine’s experience highlights the value of alternative consent methods and the need for collaboration and dialogue in addressing ethical challenges. Both Ahmed and Catherin emphasize the Neuroethics course’s role in preparing researchers for future ethical challenges.
Last Words?
Ethical research is not just a legal mandate but a commitment to the dignity and well-being of all involved. Upholding these standards goes beyond mere compliance – it is a testament to our humanity and respect for individual rights. As researchers, we must ensure that our work is conducted with integrity and respect, always prioritizing the welfare and rights ofour subjects. It’s about creating a world where research is not just about making discoveries but also about making a difference – ethically, responsibly, and with care.
So, the next time you hear about a groundbreaking research study, remember – there’s a lot more to it than just the findings. There’s a whole world of ethical considerations that made it possible. And that’s what truly makes research a noble pursuit.