OATPs
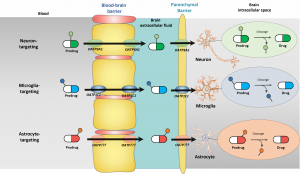
Organic anion transporting polypeptides (OATPs) can be both ubiquitously distributed within the body, and very selectively expressed in certain tissues and there are species differences in these expression patterns. From the eleven members of this family that share approximately 40% amino acid sequence identity, at least OATP1A2 (SLCO1A2), OATP1C1 (SLCO1C1), OATP2A1 (SLCO2A1), OATP2B1 (SLCO2B1), and OATP3A1 (SLCO3A1) have been detected in the brain. In addition, OATP4A1, OATP5A1, and OATP6A1 have been proposed to be expressed in the brain, however, these transporters are more or less orphans, i.e., whose functions are less well understood. More specifically, OATP1A2 and OATP2B1 are expressed at the BBB (luminal side), OATP3A1 is a neuronal transporter, OATP1C1 is an astrocytic transporter, OATP2B1 is a microglial transporter, and OATP2A1 is evenly distributed among neurons, astrocytes and neuron. This knwoledge can be further utilized rational transporter-mediated intrabrain-targeted drug delivery.
OATPs have twelve transmembrane domains (TMDs) with both termini located intracellularly. The large fifth extracellular loop has many conserved cysteine residues that can form disulfide bonds, and both the second and fifth extracellular loops contain several N-glycosylation sites. Moreover, several amino acid residues that may have crucial roles in the OATP-mediated transport have been identified. However, due to the multiple binding sites of OATPs, the detailed interactions have remained controversial. The main substrates of OATPs are anions, however, they can also carry neutral and cationic compounds. In addition, many of their substrates are amphiphilic, having hydrophilic polar features and lipophilic proportions and they are relatively large (> 350 mol/g). Most of the OATPs transport a wide variety of compounds, both endogenous as well as exogenous substrates. In general, the substrate specificities of distinct OATPs overlap, however, some of them have also very narrow and specific substrate specificities. The transport via OATPs is sodium independent, but it can be affected by the pH. In an acidic environment, the transport activity, at least some OATP subtypes, can be increased due to the increased substrate affinity via protonation of a conserved histidine residue at the extracellular end of TM3.
Selected Publications
Tonduru, A. K.; Maljaei, S. H.; Adla, S. K.; Anamea, L.; Espada1, C.; Santos, I. F.; Montaser, A. B.; Rautio, J.; Kronenberger, T.; Poso, A.; Huttunen, K. M. Targeting Glial Cells by Organic Anion Transporting Polypeptide 1C1 (OATP1C1) -Utilizing L-Thyroxine-Derived Prodrugs. Journal of Medicinal Chemistry, 2023,66(22):15094-15114. https://doi.org/10.1021/acs.jmedchem.3c01026
Tonduru, A. K.; Adla, S. K.; Huttunen, K. M.; Kronenberger, T.; Poso, A. Comparative Modelling of Organic Anion Transporting Polypeptides: Structural Insights and Comparison of Binding Modes. Molecules, 2021, 7(23), 8531. https://doi.org/10.3390/molecules27238531
Adla, S. K.; Tonduru, A. K.; Kronenberger, T.; Kudova, E.; Poso, A.; Huttunen, K. M. Neurosteroids: Structure-Uptake Relationships and Computational Modeling of Organic Anion Transporting Polypeptides (OATP)1A2. Molecules, 2021, 26(18): 5662. https://doi.org/10.3390/molecules26185662