LAT1
L-Type Amino Acid Transporter 1 (LAT1) is a pH and sodium independent antiporter. This heterodimeric protein complex is an essential carrier of large, neutral, aromatic, or branched L-amino acids (e.g., L-Leu, L-Phe, L-Tyr, L-Trp, L-His, L-Met, L-Ile, and L-Val) and some amino acid mimicking drugs (e.g., L-dopa, gabapentin, baclofen, and melphalan). LAT1 consists of a light chain (SLC7A5) and a CD98 heavy chain (4F2hc; SLC3A2), which are linked together via a disulfide bond. The exact role of CD98 is not fully understood, but it has been proposed that it chaperones the complex’s localization to the cell membrane and affects the LAT1’s transportation activity.
LAT1 is distributed throughout the body, and it is highly expressed in tissues that require a high amino acid supply, such as the brain and placenta, but it is also found to a lesser extent in other tissues. Moreover, LAT1 is needed to meet the increased demand for amino acids in proliferating cancer cells, and therefore, it is upregulated in various types of tumors. Similarly, certain immune cells have the ability to upregulate LAT1 expression on their cell surface upon their activation. The protein complex is found mainly in the basolateral membrane of polarized epithelia with an exception of the blood-brain barrier (BBB), where it is localized on both luminal and abluminal sides. It has also been found that ubiquitylation of LAT1’s N-terminal tail induces its endocytosis and degradation. Moreover, it has been demonstrated that the lysosomal protein, LAPTM4b, can recruit LAT1 to lysosomes.
We have extensively studied LAT1 as a potential carrier and target of novel drugs.
Selected Publications
Bahrami, K.; Järvinen, J.; Laitinen, T.; Reinisalo, M.; Honkakoski, P.; Poso, A.; Huttunen, K. M.; Rautio, J. Structural features affecting selectivity of LAT1-targeted phenylalanine drug conjugates. Molecular Pharmaceutics, 2022, 20(1):206-218. https://doi.org/10.1021/acs.molpharmaceut.2c00594
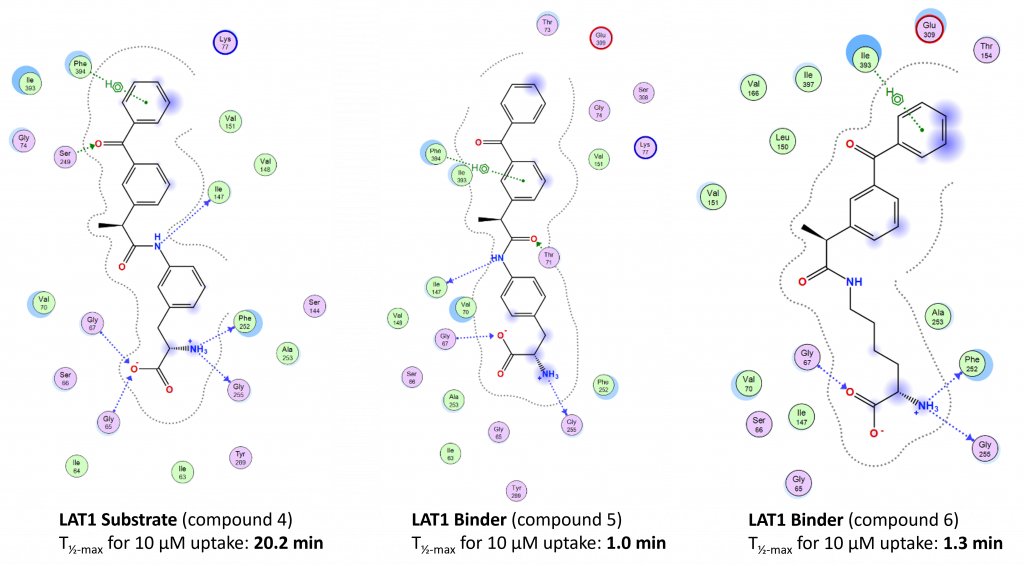
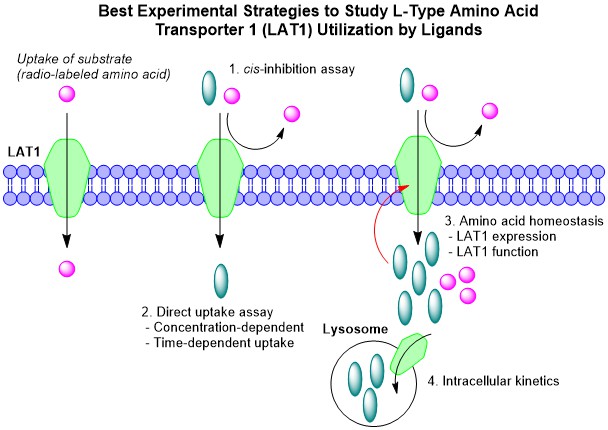
Huttunen, J.; Agami, M.; Tampio, J.; Montaser, A. B.; Huttunen, K. M. Comparison of Experimental Strategies to Study L-Type Amino Acid Transporter 1 (LAT1) Utilization by Ligands. Molecules, 2021, 27(1), 37, 37-57. https://doi.org/10.3390/molecules27010037
Jalkanen, A.; Ihalainen, J.; Lehtonen, M.; Forsberg, M. M.; Rautio, J.; Huttunen, K. M.; Gynther, M. Species differences between mice and rats in the pharmacology of a LAT1-utilizing compound. International Journal of Pharmaceutics, 2021, 596: 120300. https://doi.org/10.1016/j.ijpharm.2021.120300
Kärkkäinen, J.; Laitinen, T.; Markowicz-Piasecka, M.; Montaser, A.; Lehtonen, M.; Rautio, J.; Gynther, M.; Poso, A.; Huttunen, K.M. Molecular characteristics supporting l-Type amino acid transporter 1 (LAT1)-mediated translocation. Bioorganic Chemistry, 2021, 112: 104921. https://doi.org/10.1016/j.bioorg.2021.104921
Huttunen J.; Gynther, M.; Vellonen, K.-S.; Huttunen, K. M. L-Type Amino Acid Transporter 1 (LAT1)-Utilizing Prodrugs Are Carrier-Selective Despite Having Low Affinity for Organic Anion Transporting Polypeptides (OATPs). International Journal of Pharmaceutics, 2019, 571, 11871410. http://doi.org/10.1016/j.ijpharm.2019.118714
Kärkkäinen, J.; Gynther, M.; Kokkola, T.; Petsalo, A.; Auriola, S.; Lahtela-Kakkonen, M.; Laine, K.; Rautio, J.; Huttunen, K. M. Structural Properties for Selective and Efficient L-Type Amino Acid Transporter 1 (LAT1)- Mediated Cellular Uptake. International Journal of Pharmaceutics, 2018, 544 (1), 91-99. http://doi.org/10.1016/j.ijpharm.2018.04.025
Huttunen, K. M.; Huttunen, J.; Aufderhaar, I.; Gynther, M.; Denny, W. A.; Spicer, J. A. L-Type Amino Acid Transporter 1 (LAT1)- Mediated Targeted Delivery of Perforin Inhibitors. International Journal of Pharmaceutics, 2015, 498 (1-2), 205-216. http://dx.doi.org/10.1016/j.ijpharm.2015.12.034
Ylikangas, H.; Malmioja, K.; Peura, L.; Gynther, M.; Nwachukwu, E. O.; Leppänen, J.; Laine, K.; Rautio, J.; Lahtela-Kakkonen, M.; Huttunen, K. M.; Poso, A. Quantitative Insights for the Design of Compounds Recognized by L-Type Amino Acid Transporter 1 (LAT1). ChemMedChem, 2014, 9 (12), 2699-2707. http://dx.doi.org/10.1002/cmdc.201402281