RESEARCH
Brain glia, especially microglia and astrocytes, are in dynamic interaction with neurons and regulate neuronal functions during development and disease. Animal studies have suggested that glial dysfunctions contribute to progression of neurodegenerative diseases, such as Alzheimer’s disease (AD), or may even contribute to the disease onset. However, there is no direct evidence on whether or how the altered glial states actually impair neuronal functions in the human brain. Investigation of these events has not been possible in human tissue, since fresh human AD patient brains are not available for research. Our lab has tackled this issue by developing frameworks to link altered cellular states with neuronal operational properties in human tissue.

WINDOW TO LIVE HUMAN LIVE BRAIN
First, we have developed multimodal pipelines to evaluate, in layer and cell-type specific manner with spatial resolution, the human neuronal operational properties in surgical resections from idiopathic normal pressure hydrocephalus (iNPH) and drug-refractory epilepsy. Due to the early AD pathology present in half of the iNPH patients, iNPH patients’ brain biopsies offer a unique window to evaluate events occurring during development of early AD. By integrating extensive clinical data from the patients together with data functional, molecular and spatial data obtained from the fresh biopsies we are carrying out mechanistic investigation of how early AD-related pathology alters cellular states and functions. Investigation of biofluids (plasma/cerebrospinal fluid) also offers means for discovery of biomarkers indicative of pathology progression.
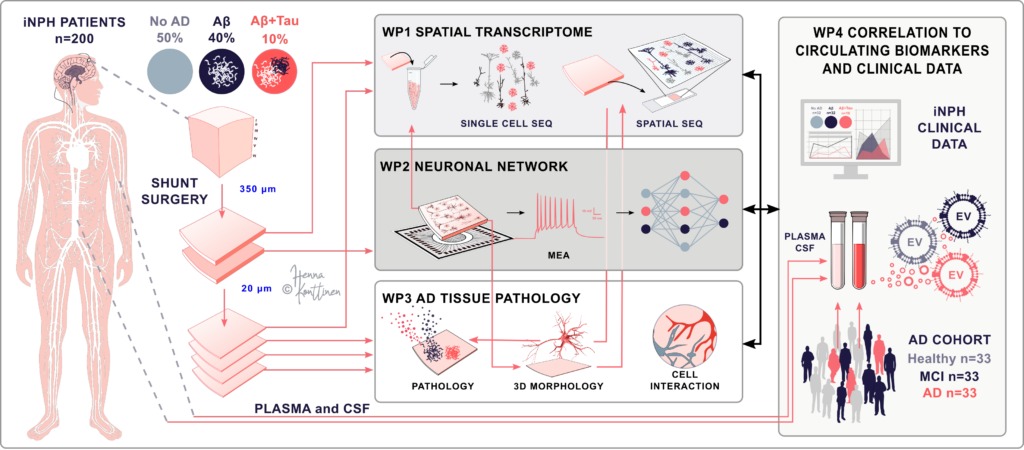
Figure. Schematic illustration of our pipeline for analyzing human brain biopsy. Image credit: Henna Jäntti.
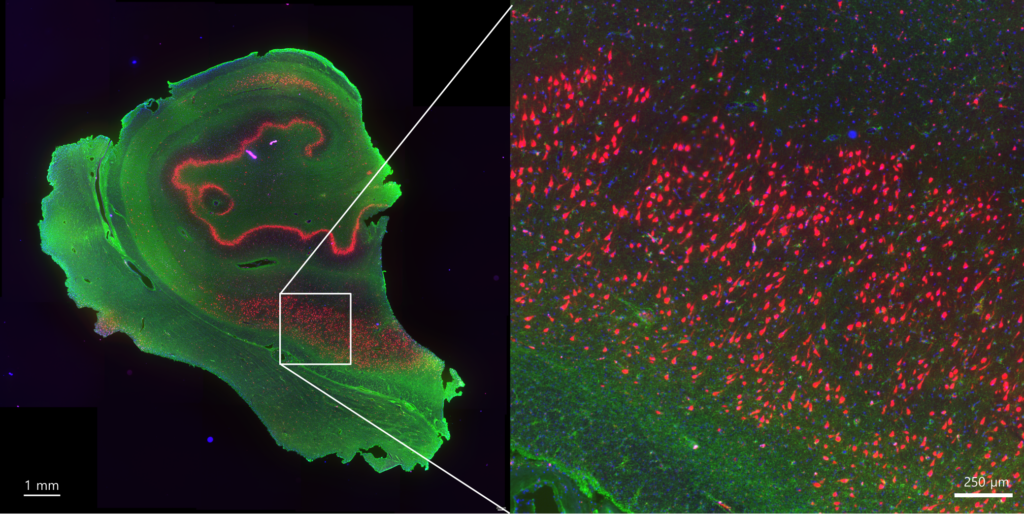
Figure. Immunohistochemistry staining of human brain sample section. Image credit: Valeriia Sitnikova.
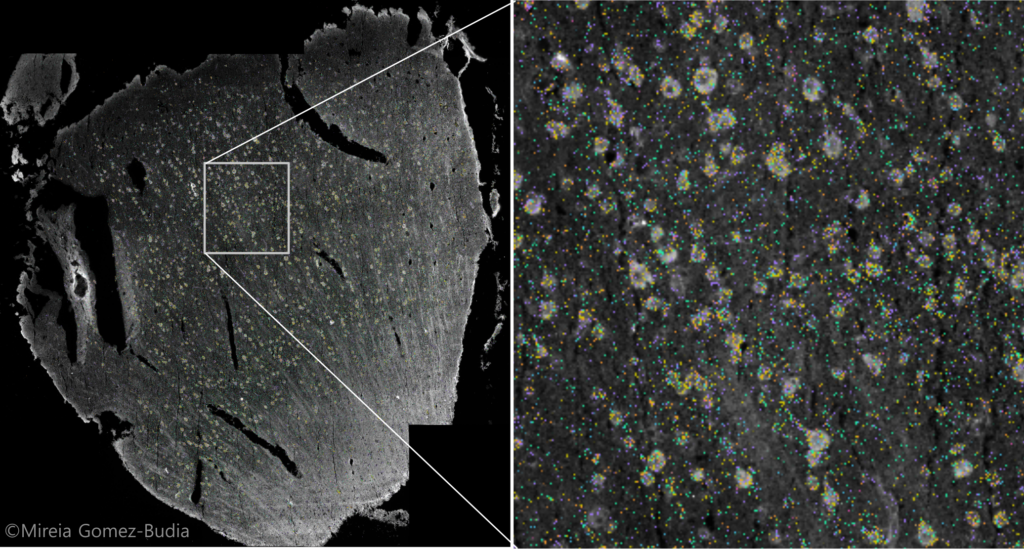
Figure. Visium 10x spatial transcriptomics of human brain sample section. Image credit: Mireia Gomez Budia.
DEVELOPMENT HUMAN STEM CELL MODELS
Second, we develop and use induced pluripotent stem cell (iPSC) -derived models, such as microglia and microglia containing organoids, to investigate microglia-neuron interaction during development of neural networks.
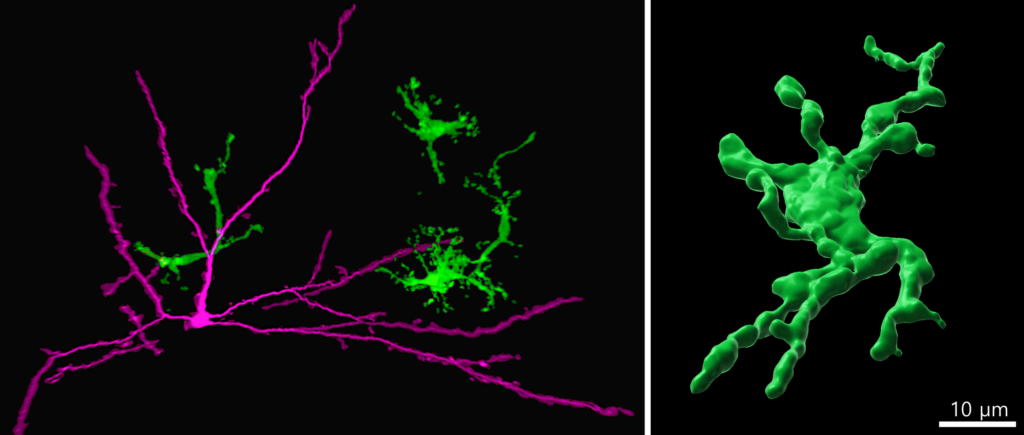
Figure. Immunohistochemistry staining of human induced pluripotent stem cell (iPSC) derived brain organoid assembloid showing human iPSC-microglia (green) interacting with neurons (magenta). Image credit: Susanne Michels.
LINKING CELLULAR STATES WITH FUNCTION
By using these tissue specimens and iPSC-derived models, we are investigating how disease pathologies, such as AD or epilepsy, alter cellular states and how the altered cellular states are linked with neuronal operational properties. Our models are also perfectly suited to investigate how different genetic variants alter microglia functions, how microglia shape the developing neuronal networks and how these events alter neuronal functions.
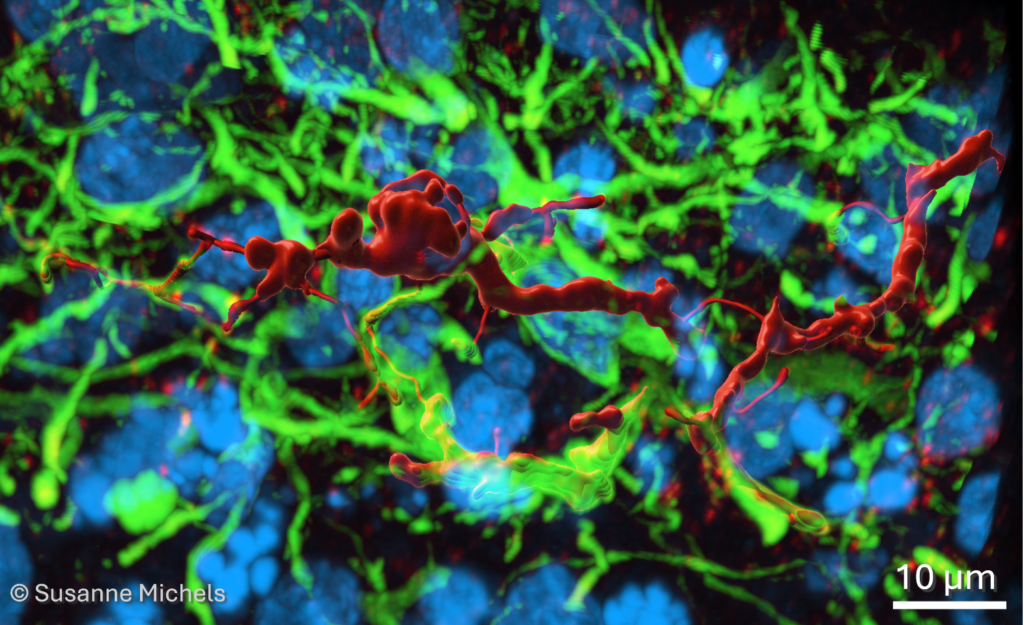
Figure. 3D reconstruction of somatostatin (red) positive inhibitory neuron in human brain organoid assembloid. Dendrites stained with MAP2 (green) and nuclei with DAPI (blue). Scale bar 10 µm. Image credit: Susanne Michels.
INTGRATING MOLECULAR AND ELECTROPHYSIOLOGICAL DATA
We combine diverse methodological approaches, such as modern molecular biology methods with patch-clamp and multielectrode array (MEA) recordings, either using the 3D 60-electrode arrays or high-density CMOS arrays. Our computational team actively develops methods to integrate different multiomic datasets with clinical data and novel pipelines for analysis of MEA data.