Biomechanics and Modeling
In osteoarthritis (OA) there are several alterations in cartilage constituents that lead to changes in the mechanical properties of cartilage; reduction of proteoglycan (PG) and collagen content, collagen fibrillation and an increase in the fluid content [1-8]. These alterations in structure and composition lead to increased permeability, allowing water to flow out of the tissue faster, and the decreased equilibrium and dynamic mechanical stiffness of articular cartilage. Most of the studies investigating structure-function relationships of cartilage [9-11] have applied microscopic, spectroscopic and biomechanical methods for the characterization of collagen and PG content, collagen orientation, and tissue mechanical stiffness, but with utilizing computational models more specific information can be obtained concerning the mechanical properties for the collagen network, PGs and fluid.
There are many computational finite element (FE) models that have been developed and applied to characterize the mechanical properties and behavior of articular cartilage [12-15], but the fibril-reinforced biphasic models are able to separate the mechanical effects of collagen, PGs and fluid in loaded articular cartilage [3,14,16-20]. In the fibril reinforced models, the nonfibrillar matrix and fibril network moduli describe the mechanical effects of PGs and collagen, while the permeability can be used to characterize fluid flow and its changes along with alterations in void ratio during tissue compression (Fig. 1).
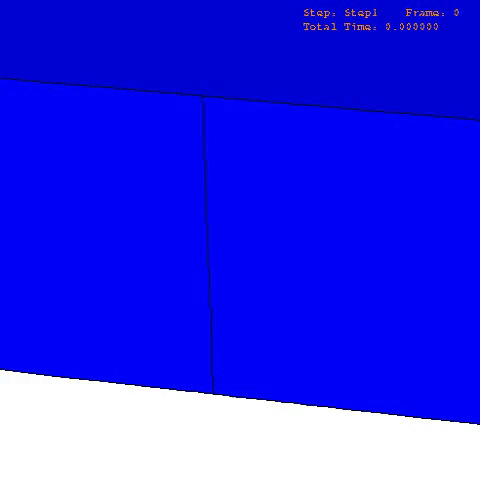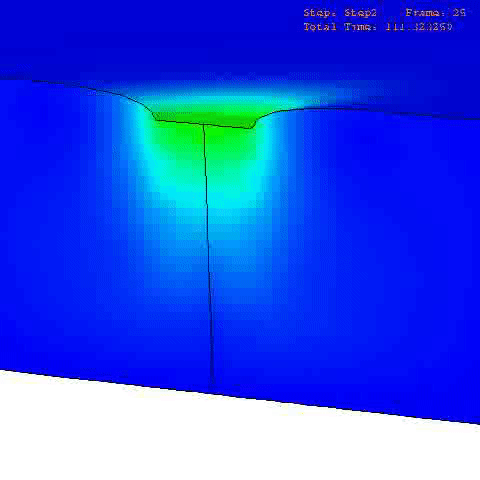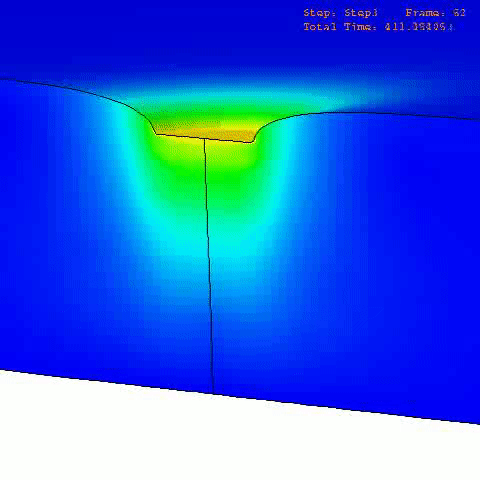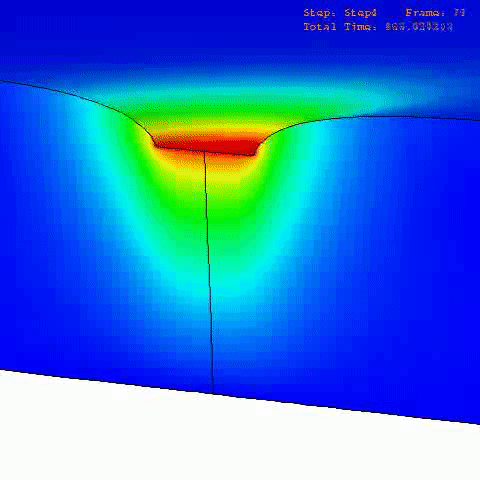
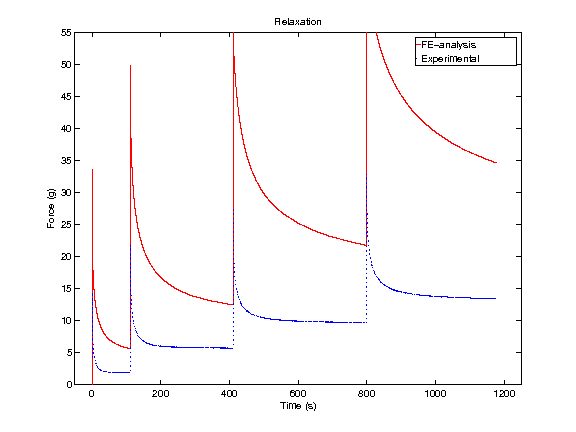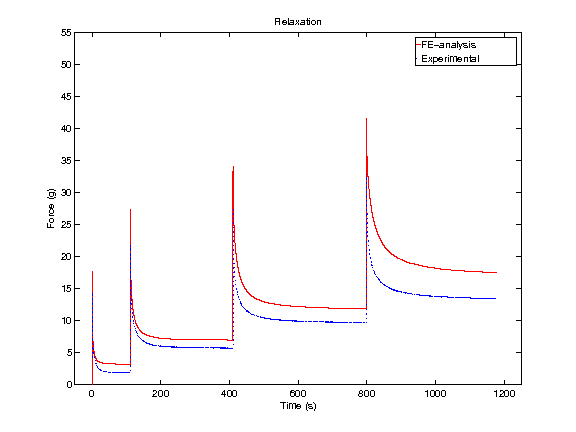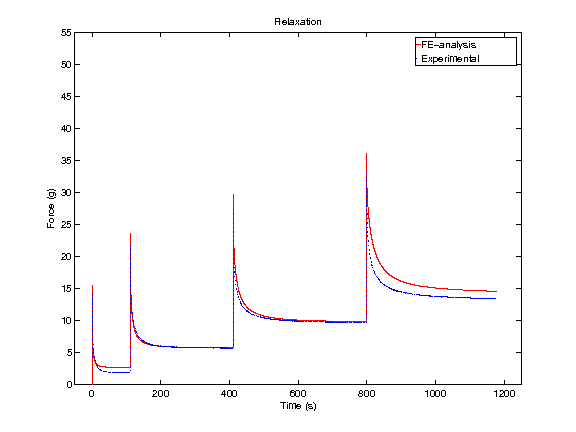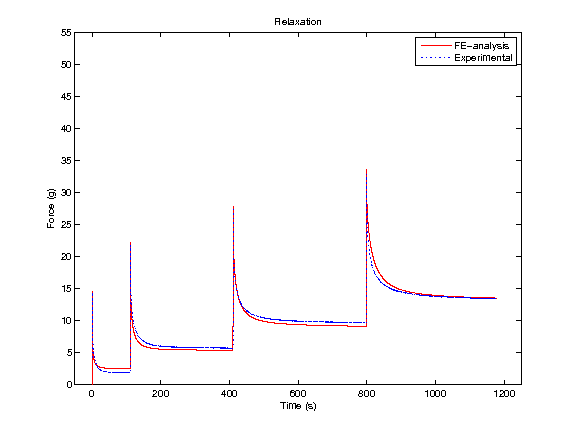
In order to obtain material parameters of cartilage, the FE model has to be fitted to the experimental biomechanical measurements (See mechanical testing equipment) by altering the material parameters of the FE model (eg., initial values of collagen and non-fibrillar network stiffness and permeability, and the strain-dependencies of these). One way of fitting can be minimizing the error between the experimental and simulated reaction forces (Fig. 2). When created FE model simulates the measured forces, the material parameters are obtained. Achieved parameters can be then used i.e., in knee joint model (See functional imaging of knee joint). When material parameters are combined with structural information, the computational methods can point out the mechanical roles of collagen, PGs and fluid and provide important and specific information of the mechanisms during the progression of OA.
References
- Arokoski JP, Jurvelin JS, Vaatainen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scand.J.Med.Sci.Sports 2000;10:186-98.
- Saarakkala S, Julkunen P, Kiviranta P, Makitalo J, Jurvelin JS, Korhonen RK. Depth-wise progression of osteoarthritis in human articular cartilage: investigation of composition, structure and biomechanics. Osteoarthritis Cartilage 2010;18:73-81. doi: 10.1016/j.joca.2009.08.003.
- Korhonen RK, Laasanen MS, Toyras J, Lappalainen R, Helminen HJ, Jurvelin JS. Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage. J.Biomech. 2003;36:1373-9.
- Buckwalter JA and Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr.Course Lect. 1998;47:487-504.
- Bi X, Yang X, Bostrom MP, Camacho NP. Fourier transform infrared imaging spectroscopy investigations in the pathogenesis and repair of cartilage. Biochim.Biophys.Acta 2006;1758:934-41. doi: 10.1016/j.bbamem.2006.05.014.
- Panula HE, Hyttinen MM, Arokoski JP, Langsjo TK, Pelttari A, Kiviranta I, et al. Articular cartilage superficial zone collagen birefringence reduced and cartilage thickness increased before surface fibrillation in experimental osteoarthritis. Ann.Rheum.Dis. 1998;57:237-45.
- Bi X, Li G, Doty SB, Camacho NP. A novel method for determination of collagen orientation in cartilage by Fourier transform infrared imaging spectroscopy (FT-IRIS). Osteoarthritis Cartilage 2005;13:1050-8. doi: 10.1016/j.joca.2005.07.008.
- Armstrong CG and Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J.Bone Joint Surg.Am. 1982;64:88-94.
- LeRoux MA, Arokoski J, Vail TP, Guilak F, Hyttinen MM, Kiviranta I, et al. Simultaneous changes in the mechanical properties, quantitative collagen organization, and proteoglycan concentration of articular cartilage following canine meniscectomy. J.Orthop.Res. 2000;18:383-92. doi: 10.1002/jor.1100180309.
- Nieminen MT, Toyras J, Rieppo J, Hakumaki JM, Silvennoinen J, Helminen HJ, et al. Quantitative MR microscopy of enzymatically degraded articular cartilage. Magn.Reson.Med. 2000;43:676-81.
- Canal Guterl C, Hung CT, Ateshian GA. Electrostatic and non-electrostatic contributions of proteoglycans to the compressive equilibrium modulus of bovine articular cartilage. J.Biomech. 2010;43:1343-50. doi: 10.1016/j.jbiomech.2010.01.021.
- Cohen B, Lai WM, Mow VC. A transversely isotropic biphasic model for unconfined compression of growth plate and chondroepiphysis. J.Biomech.Eng. 1998;120:491-6.
- DiSilvestro MR, Zhu Q, Wong M, Jurvelin JS, Suh JK. Biphasic poroviscoelastic simulation of the unconfined compression of articular cartilage: I–Simultaneous prediction of reaction force and lateral displacement. J.Biomech.Eng. 2001;123:191-7.
- Li LP, Soulhat J, Buschmann MD, Shirazi-Adl A. Nonlinear analysis of cartilage in unconfined ramp compression using a fibril reinforced poroelastic model. Clin.Biomech.(Bristol, Avon) 1999;14:673-82.
- Soltz MA and Ateshian GA. A Conewise Linear Elasticity mixture model for the analysis of tension-compression nonlinearity in articular cartilage. J.Biomech.Eng. 2000;122:576-86.
- Wilson W, van Donkelaar CC, van Rietbergen B, Ito K, Huiskes R. Stresses in the local collagen network of articular cartilage: a poroviscoelastic fibril-reinforced finite element study. J.Biomech. 2004;37:357-66.
- Julkunen P, Kiviranta P, Wilson W, Jurvelin JS, Korhonen RK. Characterization of articular cartilage by combining microscopic analysis with a fibril-reinforced finite-element model. J.Biomech. 2007;40:1862-70.
- Li LP, Buschmann MD, Shirazi-Adl A. A fibril reinforced nonhomogeneous poroelastic model for articular cartilage: inhomogeneous response in unconfined compression. J.Biomech. 2000;33:1533-41.
- Li LP, Herzog W, Korhonen RK, Jurvelin JS. The role of viscoelasticity of collagen fibers in articular cartilage: axial tension versus compression. Med.Eng.Phys. 2005;27:51-7. doi: 10.1016/j.medengphy.2004.08.009.
- Seifzadeh A, Wang J, Oguamanam DC, Papini M. A nonlinear biphasic fiber-reinforced porohyperviscoelastic model of articular cartilage incorporating fiber reorientation and dispersion. J.Biomech.Eng. 2011;133:081004. doi: 10.1115/1.4004832.
Ph.D. theses of our researchers
Rami Korhonen:
Experimental Analysis and Finite Element Modeling of Normal and Degraded Articular Cartilage
Petro Julkunen:
Relationships between Structure, Composition and Function of Articular Cartilage
Publications of our researchers
- Florea C, Malo MK, Rautiainen J, Makela JT, Fick JM, Nieminen MT, Jurvelin JS, Davidescu A and Korhonen RK. Alterations in subchondral bone plate, trabecular bone and articular cartilage properties of rabbit femoral condyles at 4 weeks after anterior cruciate ligament transection. Osteoarthritis Cartilage. 2015 Mar;23(3):414-22. doi: 10.1016/j.joca.2014.11.023.
- Danso EK, Makela JT, Tanska P, Mononen ME, Honkanen JT, Jurvelin JS, Toyras J, Julkunen P and Korhonen RK. Characterization of site-specific biomechanical properties of human meniscus-Importance of collagen and fluid on mechanical nonlinearities. J Biomech. 2015 Feb 7. pii: S0021-9290(15)00064-0. doi:
- Makela JT, Rezaeian ZS, Mikkonen S, Madden R, Han SK, Jurvelin JS, Herzog W and Korhonen RK. Site-dependent changes in structure and function of lapine articular cartilage 4 weeks after anterior cruciate ligament transection. Osteoarthritis Cartilage. 2014 Jun;22(6):869-78. doi: 10.1016/j.joca.2014.04.010.
- Makela JT, Huttu MR and Korhonen RK. Structure-function relationships in osteoarthritic human hip joint articular cartilage. Osteoarthritis Cartilage. 2012 Nov;20(11):1268-77. doi:
- Mononen ME, Julkunen P, Toyras J, Jurvelin JS, Kiviranta I and Korhonen RK. Alterations in structure and properties of collagen network of osteoarthritic and repaired cartilage modify knee joint stresses. Biomech Model Mechanobiol. 2011 Jun;10(3):357-69. Epub 2010 Jul 14.
- Julkunen P, Wilson W, Jurvelin JS and Korhonen RK. Composition of the pericellular matrix modulates the deformation behaviour of chondrocytes in articular cartilage under static loading. Med Biol Eng Comput. 2009 Dec;47(12):1281-90. Epub 2009 Nov 7.
- Saarakkala S, Julkunen P, Kiviranta P, Makitalo J, Jurvelin JS and Korhonen RK. Depth-wise progression of osteoarthritis in human articular cartilage: investigation of composition, structure and biomechanics. Osteoarthritis Cartilage. 2010 Jan;18(1):73-81. Epub 2009 Aug 29.
- Julkunen P, Jurvelin JS and Isaksson H. Contribution of tissue composition and structure to mechanical response of articular cartilage under different loading geometries and strain rates. Biomech Model Mechanobiol. 2010 Apr;9(2):237-45. Epub 2009 Aug 13.
- Julkunen P, Harjula T, Iivarinen J, Marjanen J, Seppanen K, Narhi T, Arokoski J, Lammi MJ, Brama PA, Jurvelin JS and Helminen HJ. Biomechanical, biochemical and structural correlations in immature and mature rabbit articular cartilage. Osteoarthritis Cartilage. 2009 Dec;17(12):1628-38. Epub 2009 Jul 8.
- Julkunen P, Wilson W, Jurvelin JS, Rieppo J, Qu CJ, Lammi MJ and Korhonen RK. Stress-relaxation of human patellar articular cartilage in unconfined compression: prediction of mechanical response by tissue composition and structure. J Biomech. 2008;41(9):1978-86. Epub 2008 May 19.
- Julkunen P, Korhonen RK, Nissi MJ and Jurvelin JS. Mechanical characterization of articular cartilage by combining magnetic resonance imaging and finite-element analysis: a potential functional imaging technique. Phys Med Biol. 2008 May 7;53(9):2425-38. Epub 2008 Apr 17.
- Julkunen P, Korhonen RK, Herzog W and Jurvelin JS. Uncertainties in indentation testing of articular cartilage: a fibril-reinforced poroviscoelastic study. Med Eng Phys. 2008 May;30(4):506-15. Epub 2007 Jul 12.
- Julkunen P, Kiviranta P, Wilson W, Jurvelin JS and Korhonen RK. Characterization of articular cartilage by combining microscopic analysis with a fibril-reinforced finite-element model. J Biomech. 2007;40(8):1862-70. Epub 2006 Oct 18.
- Kiviranta P, Rieppo J, Korhonen RK, Julkunen P, Toyras J and Jurvelin JS. Collagen network primarily controls Poisson’s ratio of bovine articular cartilage in compression. J Orthop Res. 2006 Apr;24(4):690-9.
- Laasanen MS, Toyras J, Korhonen RK, Rieppo J, Saarakkala S, Nieminen MT, Hirvonen J and Jurvelin JS. Biomechanical properties of knee articular cartilage. Biorheology. 2003;40(1-3):133-40.
- Korhonen RK, Wong M, Arokoski J, Lindgren R, Helminen HJ, Hunziker EB and Jurvelin JS. Importance of the superficial tissue layer for the indentation stiffness of articular cartilage. Med Eng Phys. 2002 Mar;24(2):99-108.